Background: Prognostic modeling allows personalized risk prediction for individual patients (pt). The A-HIPI model in advanced stage classical Hodgkin lymphoma (AS-HL), developed and validated by the HoLISTIC Consortium (www.hodgkinconsortium.org), generates the individualized probability of a progression-free survival (PFS) event or death (OS) within the first 5 years (y) from diagnosis in pts based on continuous variables (www.qxmd.com/calculate/calculator_869/a-hipi). Clinical prognostic tools in lymphoma (eg, IPS, IPI, FLIPI, etc) typically use groupings of categorical values to define risk. Grouping a continuous value often results in loss of information, and most tools are not predictive for individual pts. However, discrete groupings have clinical utility & practicality for a) defining pt populations for clinical trials & real world studies, b) stratification within clinical trials, and c) crafting treatment guidelines. We studied potential approaches for utilizing the A-HIPI model to generate risk groups with input on strengths & limitations from the HoLISTIC modeling team & clinical experts.
Methods: The A-HIPI model was developed via TRIPOD guidelines on 4,022 pts treated on 8 international clinical trials for AS-HL (Rodday. JCO 2023). External validation was performed on a dataset of 1,431 pts from 4 prospective registries. The 5y PFS (PFS5) in the A-HIPI development dataset was 77% (95% CI: 76-78); the 5y OS (OS5) was 92% (95% CI: 91-93). This represented the average outcome for a pt with AS-HL pt naïve to other clinical data at presentation. The distribution of PFS5 & OS5 predictions were heavily skewed (ie, asymmetric distribution) in both the A-HIPI discovery and validation dataset. While not unexpected due to the excellent PFS & OS in this disease setting, this presents challenges in the delineation of risk groups as depicted below . Three approaches were examined for the generation of A-HIPI risk groups. Proposed cutoffs were defined using the distribution of A-HIPI risk scores and data from the model-building cohort (ie, clinical trials). Validation was done using the A-HIPI validation cohort (ie, HL registries).
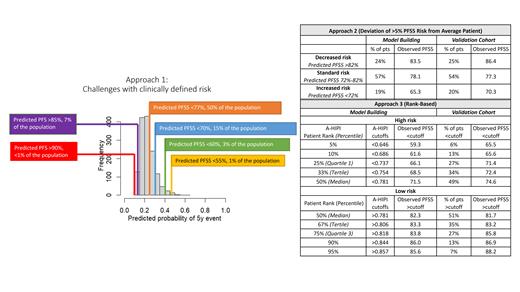
Results: Approach 1: Risk groups based on clinical thresholds. Clinicians were queried what estimates of PFS5 would constitute high vs low risk. The positive, right-skewed distribution of A-HIPI risk scores limited this approach ( Figure), as cutoffs of PFS5 <70 and PFS5> 90 would only identify 15% and <1% of the population, respectively.
Approach 2: Risk groups based on deviation from “average” pt. The 5-y PFS was 77% (95% CI: 76-78). We explored defining “standard risk” based on this confidence interval as well as clinical boundaries, with pts above or below this classified as decreased or increased risk, respectively. Results of a +/-5% clinical boundary are presented in the Table.
Approach 3: Risk groups based on “ranking” of pts. Here we ranked the A-HIPI risk scores of the 4022 AS-HL pts in the model building cohort and used the distribution of the risk scores as a benchmark. The risk profile for a future pt is then compared to this distribution (eg, how do you rank compared to your peers). Continuous results are often presented this way (eg, tertiles or quartiles). It also allows flexibility for the user to define the size of the risk groups and/or clinical threshold of interest. Application of this approach showed good alignment between the predicted model percentiles and the observed distribution of scores in the validation cohort ( Table). This is also reflected in calibration curves presented in the primary manuscript. An online application (eg, R-Shiny) will be provided at the meeting to allow users to define their own cutoffs to aid in pt prognostication as well as identify populations for clinical trial development.
Conclusions: There are challenges with defining risk groups from individual risk prediction modeling in AS-HL. Different applications and purposes, the skewed distribution of events/risk estimates, as well as varying clinical definitions of high risk, make it challenging to define consensus expert-based risk groupings for AS-HL. A flexible “rank-based” approach appeared to provide the most clinical utility & data granularity, which may be leveraged for clinical trial design and pt stratification. Further analysis and discussion of how to optimally define high-risk and low-risk populations in AS-HL will be needed as new therapeutic options emerge.
Disclosures
Maurer:Roche/Genentech: Research Funding; BMS: Consultancy, Research Funding; GenMab: Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive Biotechnology: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Morphosys: Research Funding. Parsons:Seagen: Consultancy. Hawkes:Antengene: Membership on an entity's Board of Directors or advisory committees; Merck Sharpe & Dohme: Membership on an entity's Board of Directors or advisory committees; Merck KgA: Research Funding; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses, Research Funding, Speakers Bureau; Gilead: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Other; Beigene: Other; Specialised Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Regeneron: Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding. Savage:BMS: Consultancy, Honoraria, Research Funding; Merck: Consultancy, Honoraria; Astra Zeneca: Consultancy, Honoraria; Roche: Research Funding; Seagen: Honoraria; Abbvie: Consultancy, Honoraria; Janssen: Consultancy, Honoraria. Zinzani:TAKEDA: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; GILEAD: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ROCHE: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; INCYTE: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ASTRAZENECA: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BEIGENE: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; JANSSEN-CILAG: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; EUSAPHARMA: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; MSD: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SECURA BIO: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SERVIER: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; NOVARTIS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SANDOZ: Membership on an entity's Board of Directors or advisory committees; KYOWA KIRIN: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CELLTRION: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ADC THERAPEUTICS: Membership on an entity's Board of Directors or advisory committees. Evens:Pharmacyclics: Consultancy, Honoraria; Epizyme: Consultancy, Honoraria; Morphosys: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Daiichi Sankyo: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria; Novartis: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal